Background: Improving the outcomes of light chain (AL) amyloidosis patients and advanced heart involvement, IIIb Mayo cardiac stage, is an unmet need. The standard front-line treatment has been cyclophosphamide-bortezomib-dexamethasone (CyBorD) until the approval of daratumumab with CyBorD (Dara-CyBorD) in 2021, which significantly improved the rate of hematologic response (HR) as reported by the ANDROMEDA trial. However, stage IIIb patients were excluded from the study and the best upfront treatment strategy in these patients remains to be clarified. For this reason, daratumumab was not licensed for use in stage IIIb patients in many countries. Preliminary data of the ongoing EMN22 phase II multicenter study (NCT04131309), that specifically addresses this issue using daratumumab monotherapy, are promising. Chakraboty et al. reported that Dara-CyBorD is effective in inducing profound hematologic response, at least a very good partial response (VGPR), in treatment-naïve patients with IIIb AL amyloidosis. Similar results were reported by Theodorakakou et al. using different daratumumab combinations. Finally, in a study conducted by Oubari et al. daratumumab-based regimens were associated with better clinical outcomes compared to others treatments. However, all available data on daratumumab in stage IIIb patients derive from uncontrolled studies or lacked a matched or stratified control group. We designed the present retrospective case-control study to assess the efficacy of daratumumab-based therapies versus CyBorD regimens as upfront treatment for newly diagnosed AL amyloidosis with IIIb cardiac stage.
Methods:the whole study population comprises 62 matched newly diagnosed patients with IIIb stage AL amyloidosis evaluated in our center between 2012 and 2022. The daratumumab cohort is composed of all the 31 consecutive subjects treated with daratumumab-based regimens between 2021 and 2022. Thirteen patients (41%) received daratumumab in combination with bortezomib [11 (35%) associated with melphalan and dexamethasone; 2 (6%) Dara-CyBorD], 11 (35%) with lenalidomide and 7 (22%) were treated with daratumumab monotherapy. They were matched with 31 controls selected from a total of 71 subjects treated with CyBorD. Patients and controls were matched for age, estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide, free light chain and bone marrow plasma cell. Hematologic response was assessed according to the International Society of Amyloidosis criteria. The analysis of response was by intent-to-treat: patients who died before response evalutation were considered non responders . PFS is defined as the time from diagnosis to any one of the following events, whichever comes first: death, starting a second line of therapy, organ or hematologic progression as per consensus guidelines.
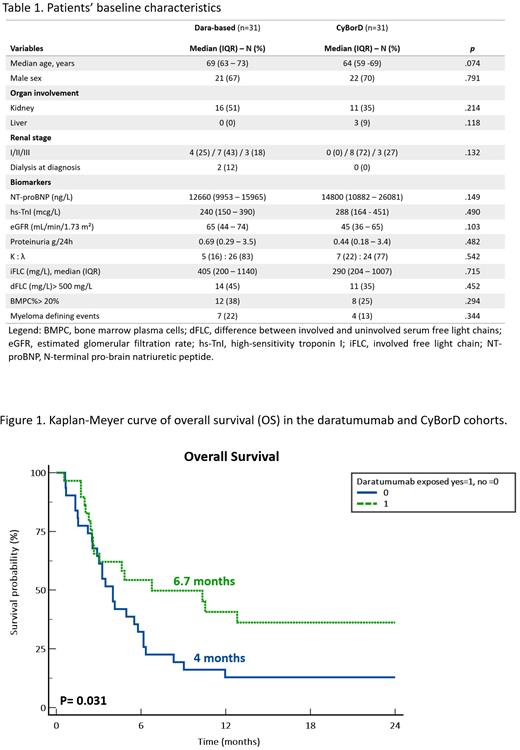
Results:Patients' characteristics are listed in Table 1. Median follow-up of living patients was 20.5 months (95% CI: 14.3 - 55.9 months). Median OS was 4.6 months (95% CI: 3 - 6.3 months), and patients of the daratumumab cohort had a significantly prolonged OS (6.7 vs 4 months; p=0.032, Fig. 1). No differences in early mortality rate, within 3 months from diagnosis, were observed between the two cohorts (32% vs 35%, p=0.796). At three months, the overall HR rate to daratumumab was 50% [complete response (CR) 12%, VGPR 29%, partial response (PR) 9%] as compared with 21% (CR 6%, VGPR 9%, PR 6%) of the control group, p=0.021. A higher proportion of ≥VGPR was observed in the daratumumab cohort (41% vs 16%; p=0.029). Cardiac response rate was significantly more frequent in patients treated with daratumumab [8 (25%), 3 cardiac VGPR and 5 cardiac PR] compared to controls [1 (3%) cardiac PR], p=0.014. A significantly prolonged PFS was observed in patients receiving daratumumab compared to CyBorD (5 vs 2.4 months; p=0.017).
Conclusions: This matched case-control study shows that even in stage IIIb patients the addition of daratumumab to CyBorD results in higher rates of deep hematologic response. This is associated with higher cardiac response rates and prolonged OS and PFS. Although the outcome of stage IIIb patients remains dismal, Dara-CyBorD was able to improve response rate and survival in these severely compromised subjects. Taken together these observations encourage the use of daratumumab combinations also in stage IIIb patients with AL amyloidosis.
Disclosures
Basset:Jannsen: Honoraria. Nuvolone:Jannsen: Honoraria. Foli:Jannsen: Honoraria. Palladini:Sebia: Honoraria; Prothena: Consultancy, Honoraria; Pfizer: Honoraria; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Argobio, GSK: Consultancy; Siemens: Honoraria. Milani:Pfizer: Honoraria; Jannsen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees; Siemens: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal